
I attended The 2023 Burrow Lecture in Endocrinology, given by Professor Samuel Klein on metabolically healthy obesity. I randomly saw the flier above on Twitter, and immediately marked my calendar to attend.
I’m interested in the topic of obesity given
- My personal love and interest in health and wellness
- the disturbing rise of obesity, in America and worldwide
- the explosive debut of obesity drugs like Ozempic and the incredible effects they might have on society
I am not a medical professional, and admittedly a lot of this flew right over my head. Human biology is incredibly complex and the talk was designed for people with extensive medical training.
Still, I found the experience educational and valuable, with lots of interesting learnings. I’ve distilled some of the most interesting of those learnings below. Please note this does not cover the entirety of Professor Klein’s lecture.
Background
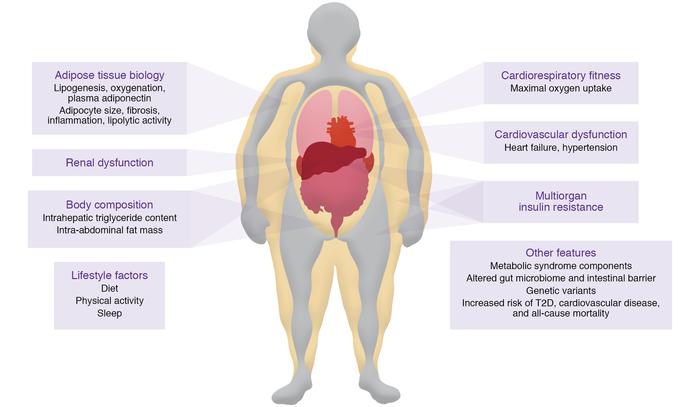
Obesity almost always drives poor metabolic health outcomes, and we still don’t know why.
There are outliers in the population — the metabolically healthy obese — that seem to show no metabolic consequences of their obesity.
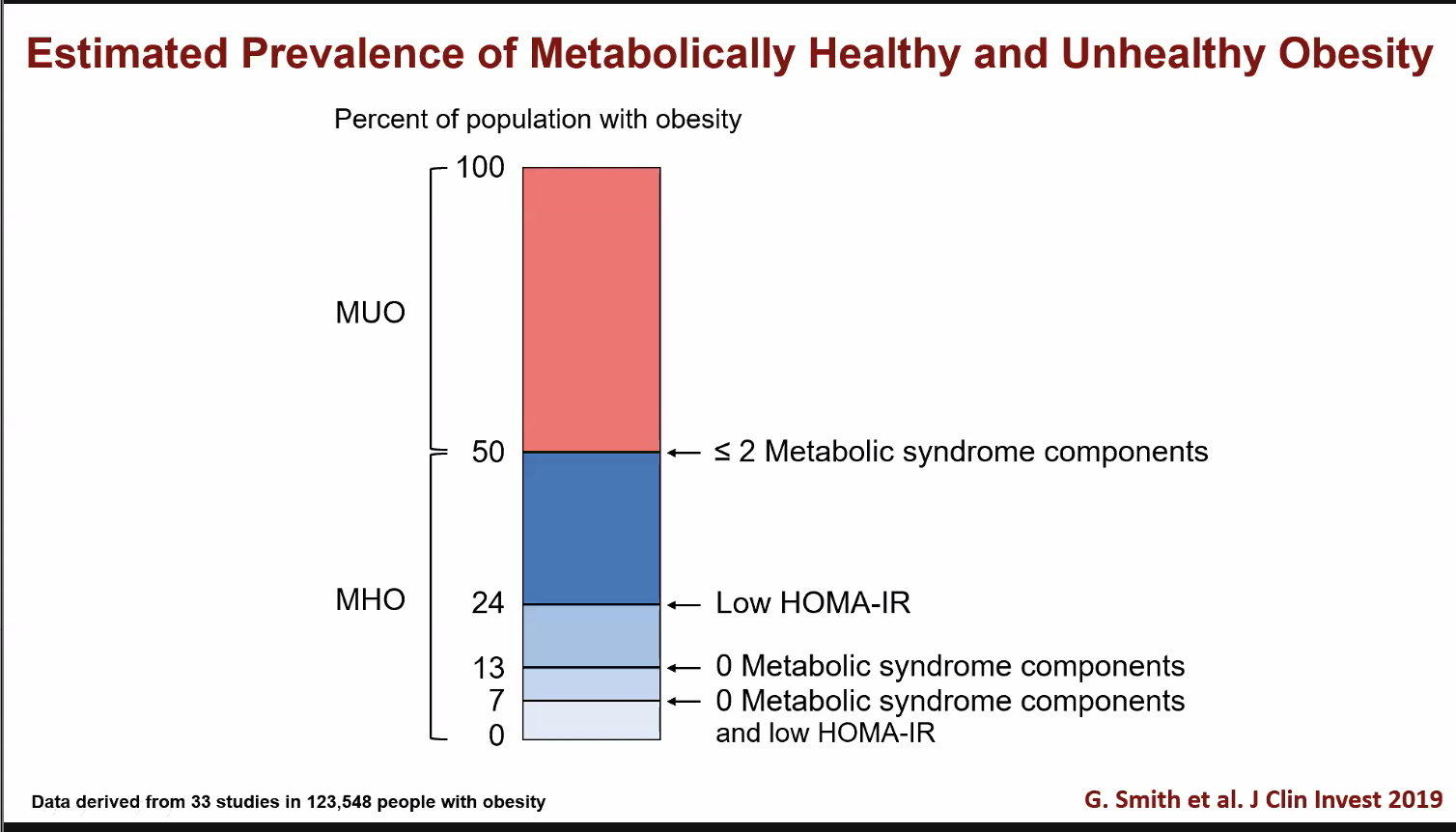
MUO = Metabolically Unhealthy Obese, MHO = Metabolically Healthy Obese
As of 2019, the medically healthy obese population was estimated to be roughly 5% by the most rigorous definition and 50% by the least strict definition.
One thing that’s problematic is that there is a lot of variability in how we define metabolically healthy obesity. You can see this in the graph above—in the least strict definition, it’s possible to have Type 2 Diabetes and still be metabolically healthy obese.
More women are likely to be healthy obese than men, and as you age the likelihood of you staying metabolically healthy decreases.
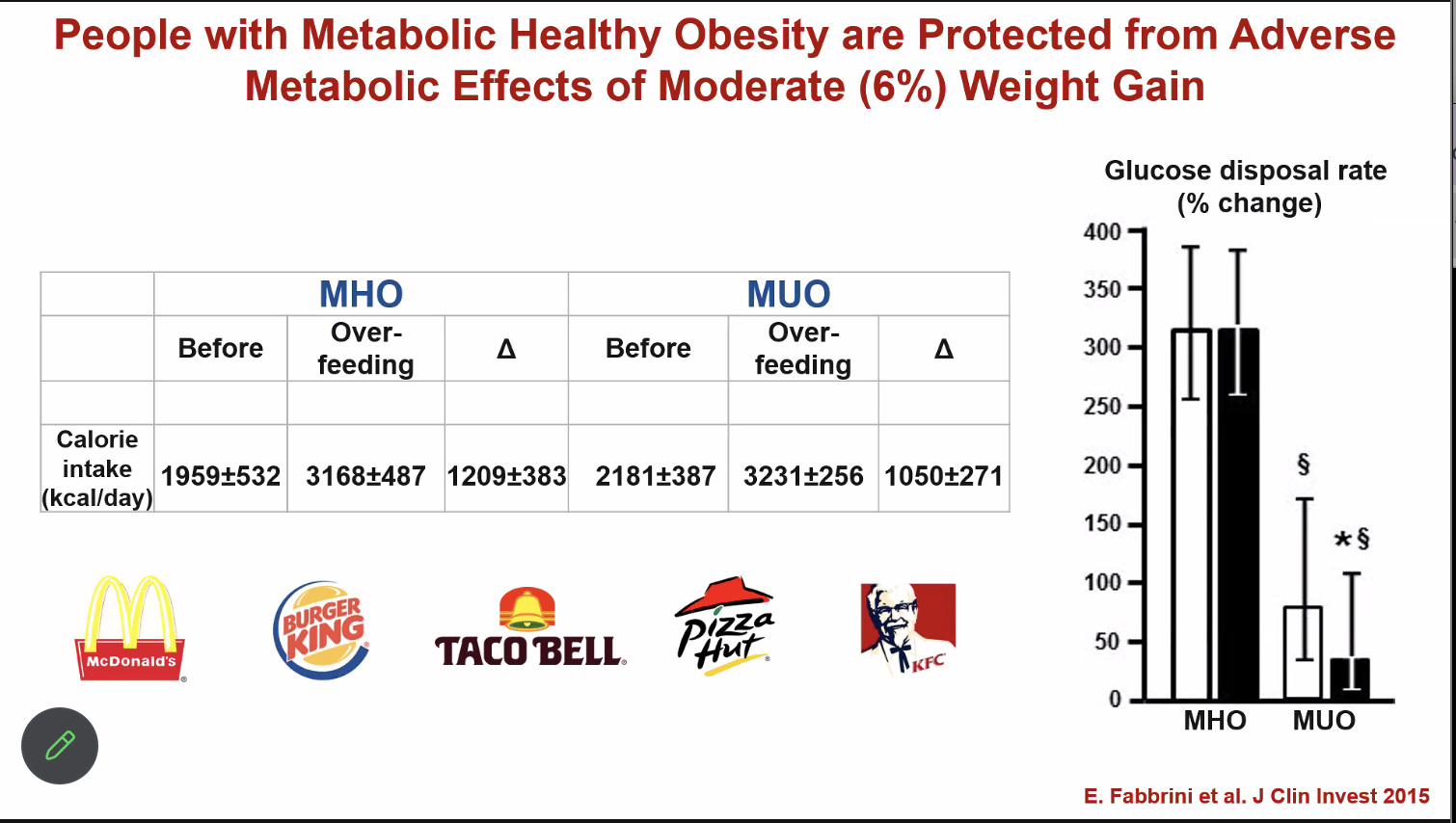
When the metabolically healthy obese were overfed, there was no change in glucose disposal, in contrast to the metabolically unhealthy obese.
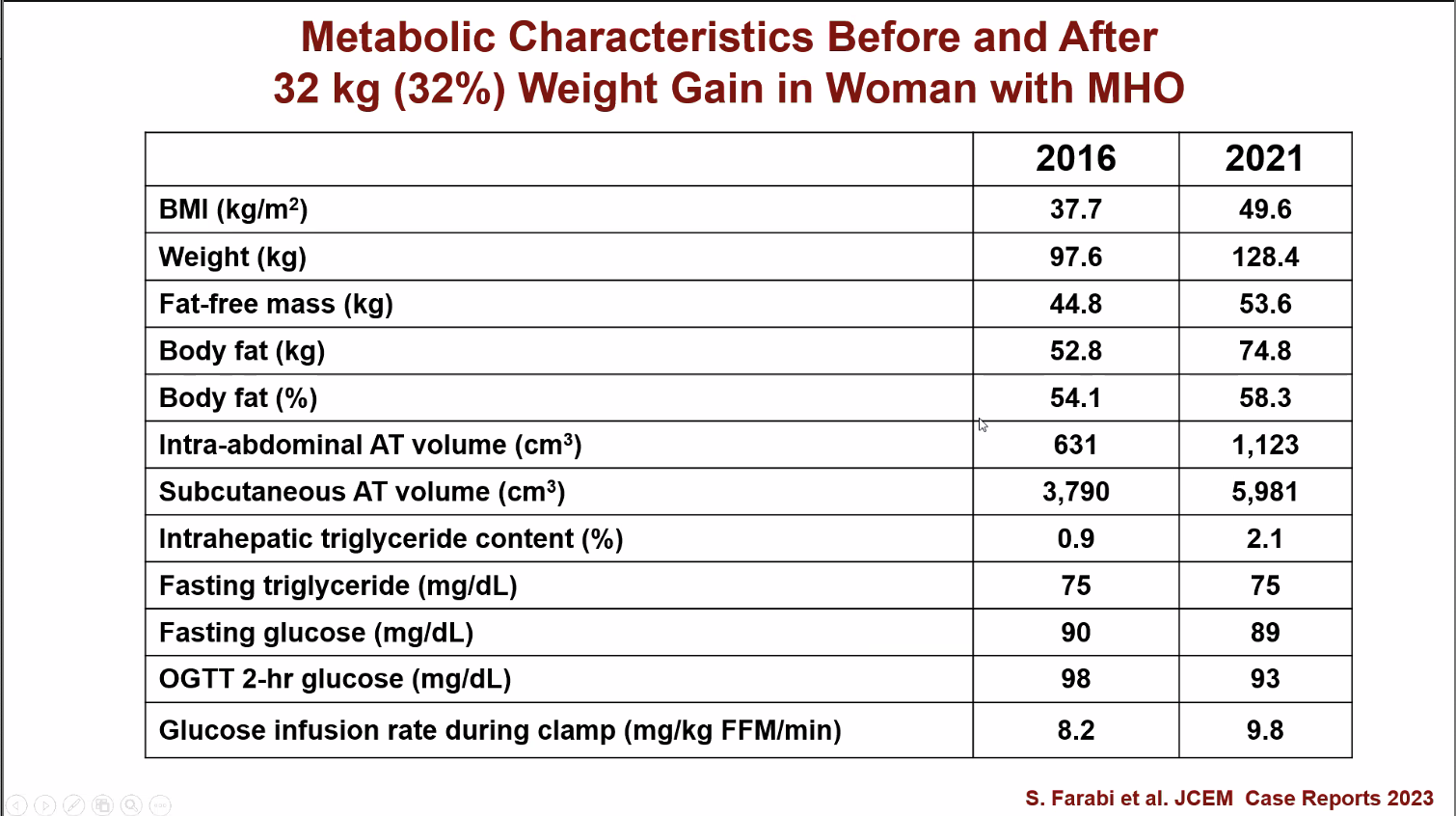
This woman gained 32% bodyfat with very little change in some of her metabolic health markers!
“If it’s not broken, you can’t fix it.” Modest weight gain doesn’t change much for the metabolically healthy obese, and crucially, weight loss doesn’t seem to drive improved outcomes, if they go on to develop metabolic issues.
If we learn more about the causes of metabolically healthy obesity and standardize the definition, we could use this to help wisely allocate resources like weight loss drugs.
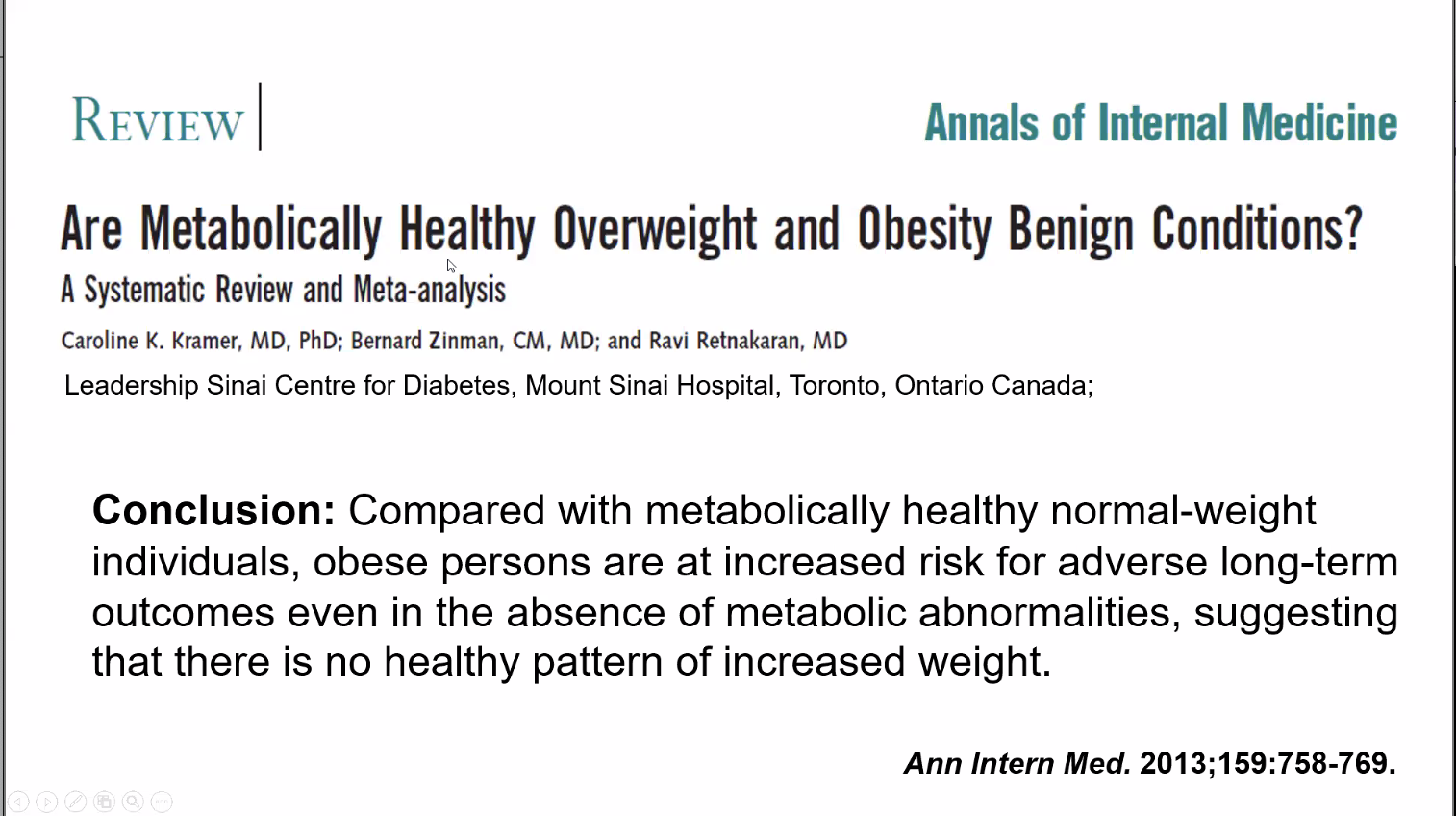
Finally, this is not a get-out-of-jail free card. Obesity drives long term adverse outcomes independent of metabolic health.
Why does being obese affect your metabolic health?
There’s quite a few theories why being obese drives poor metabolic whole body adverse outcomes, but we are not close to a decided upon answer.
Expandability hypothesis
Fat isn’t just the byproduct of excess food intake — it’s an important organ in our bodies and necessary to thrive, playing roles in the immune system, hormone regulation, and nervous system function. There are different types of fat cells (white and brown) and fat storage locations (subcutaneous, found under the skin and visceral, found around the organs).
When you gain weight, fat cells grow both in number (hypertrophic growth) and in size (hyperplastic growth).
The expandability hypothesis suggests that as you gain weight, your fat cells grow larger in size, and poor metabolic health occurs as a result of the overly large fat cells. It’s clear that obese people do in fact have larger fat cells, but how that drives metabolic health consequences is not settled science.
Why would big fat cells drive poor metabolic health outcomes?
- The larger fat cells could require more blood flow, and if the individual is unable to provide that, it triggers a cascading effect of poor outcomes.
- Or, when the fat cell size limit is reached, excess fat spills over into the organs, causing conditions like non-alcoholic fatty liver disease.
Differences in cell biology
There are fundamental differences in the biology of fat cells in the obese, metabolically healthy obese, and the metabolically lean beyond just sheer size, and these differences may contribute to negative health outcomes as well.
Oxygen limitation
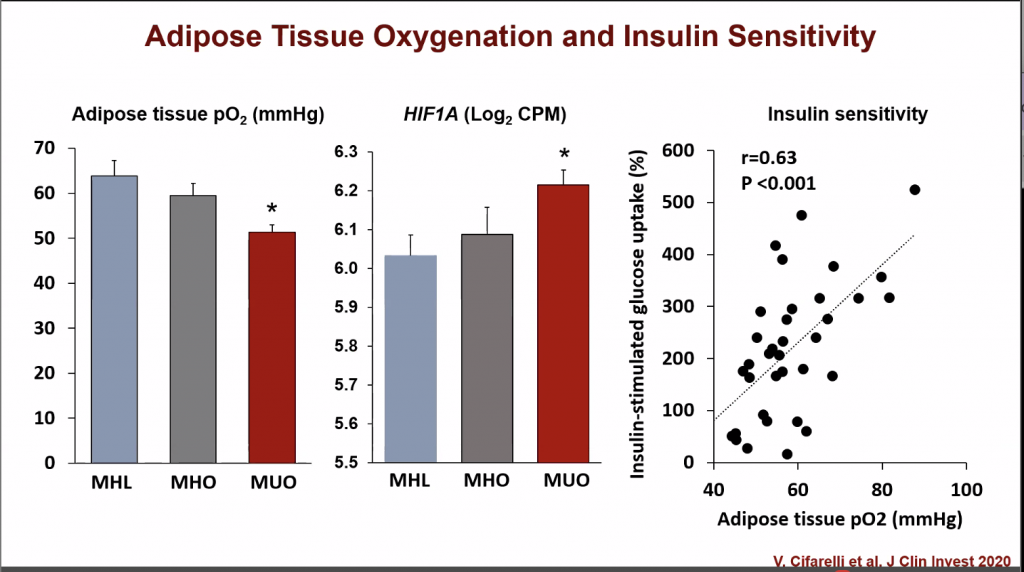
This study took a look at three groups of people who were carefully chosen to represent different levels of body fat and insulin sensitivity. These groups were:
- Metabolically healthy lean (MHL): People who were lean and didn’t have insulin resistance.
- Metabolically healthy obese (MHO): People who were obese but didn’t have insulin resistance.
- Metabolically unhealthy obese (MUO): People who were obese and had insulin resistance.
When studying the oxygenation of the fat cells of these three populations, the MUO had the least oxygen in their fat tissue, and the highest levels of HIF1A gene expression, a gene which encodes a protein that plays a role in our body’s response to low oxygen levels.
The insufficient oxygen in the fat cells in the MUO in turn sparks other changes that lead to insulin resistance. A specific protein in the blood, called PAI-1, increases when fat tissue oxygen is low.
It’s hypothesized that PAI-1 may interfere with insulin signaling pathways. Having too much of PAI-1 makes your body less sensitive to insulin, driving insulin resistance.
Companies and drugmakers are hesitant to research or interfere with PAI-1 to treat obesity, because the protein’s first and foremost job in our body is to monitor blood clots that occur as a part of healing.
PAI-1 “monitors” your scabs, basically, and makes sure they don’t get messed up before healing occurs. Companies feel the risk of interfering with the “clotting cascade” is too great to investigate PAI-1 for drugs.
Increased collagen
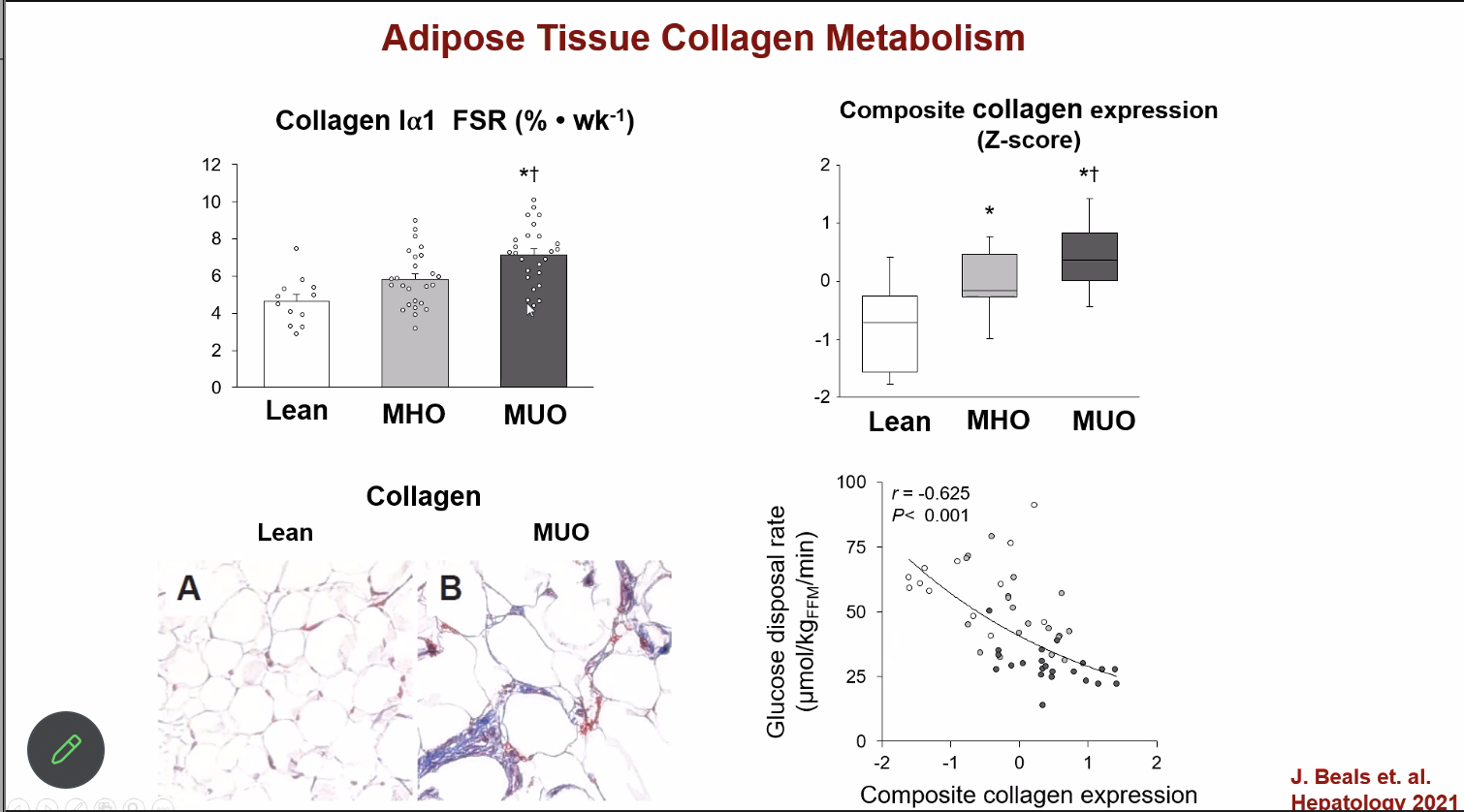
Another difference in biology between the obese and the lean is collagen production. In this 2021 study, it was demonstrated that the MUO produced collagen faster and had more genes turned on that make collagen compared to the other groups.
This increased collagen production was correlated with decreased insulin sensitivity.
We can see these clear differences in collagen and collagen production, but don’t know exactly why they drive whole body systemic differences. There’s some preliminary research that is starting to tease out why, but it needs more work.
Inflammation and regulation of exosomes
Exosomes are tiny packets that cells release, which can carry information or signals to other cells—they are kind of like a communication program between organs. They can do tons of different things and influence lots of cell activities–more than more traditionally thought of signals like hormones.
The metabolically unhealthy obese have way more exosomes than lean people do, and researchers are just starting to find differences in those exosomes that may contribute to poor metabolic health. For example, one team from China isolated specific proteins in exosomes that obese individuals have more of — proteins that are involved in the insulin signaling pathway.
In the below study summarized by Professor Klein, researchers studied three groups:
- Thin people with normal liver fat (metabolically healthy lean, MHL)
- Obese people with normal liver fat (metabolically healthy obese, MHO)
- Obese people with NAFLD (metabolically unhealthy obese, MUO)
They checked for signs of inflammation in the belly fat, measured how well the body used insulin, and studied the effects of the exosomes on muscle and liver cells.
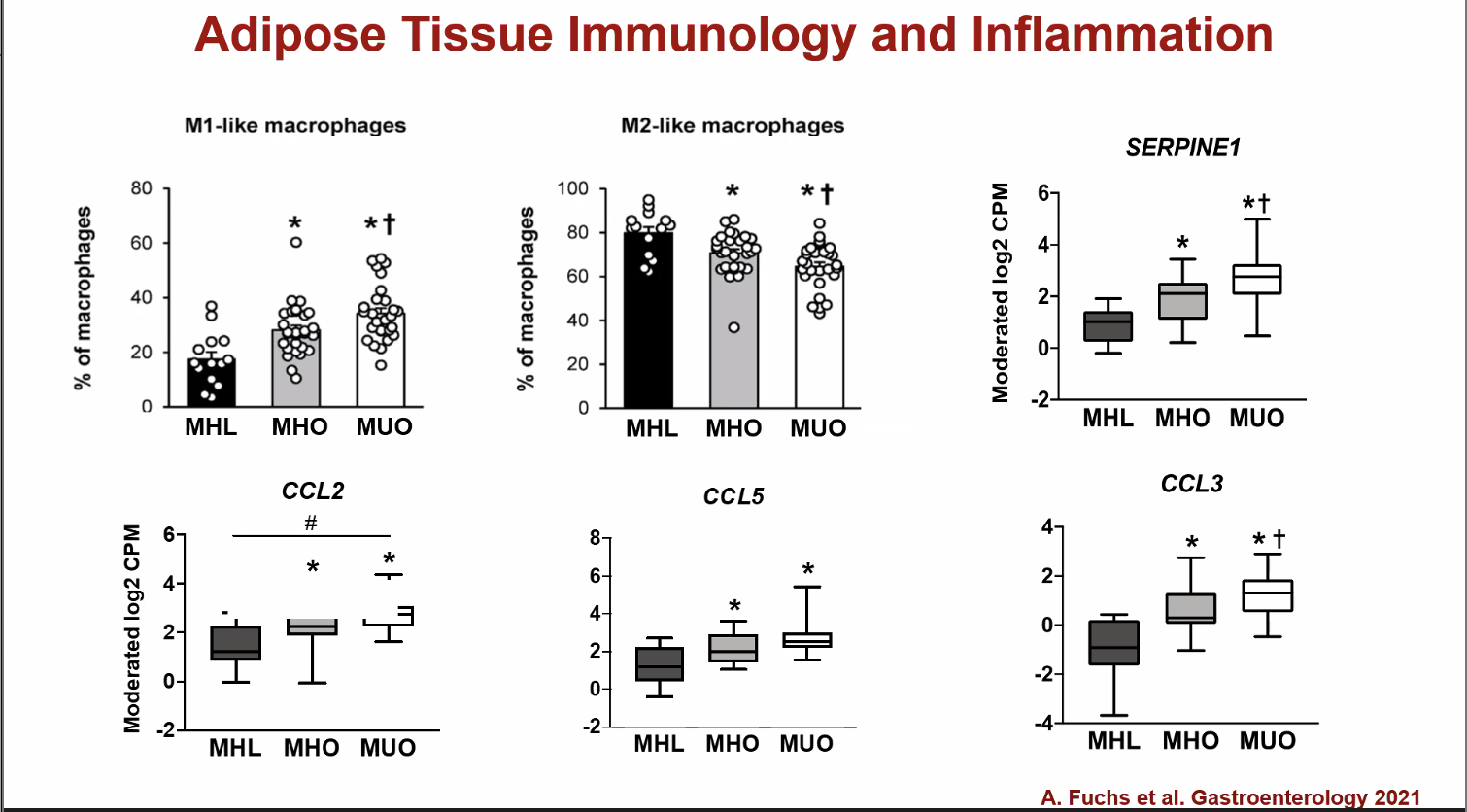
M1-like macrophages are pro-inflammatory white blood cells, the number of which increases in the metabolically healthy obese, and increases even more in the metabolically unhealthy obese. This relationship is inversed for the M2-like macrophages, or the anti-inflammatory white blood cells. SERPINE1, CCL2, CCL5, and CCL3 are gene markers associated with inflammation.
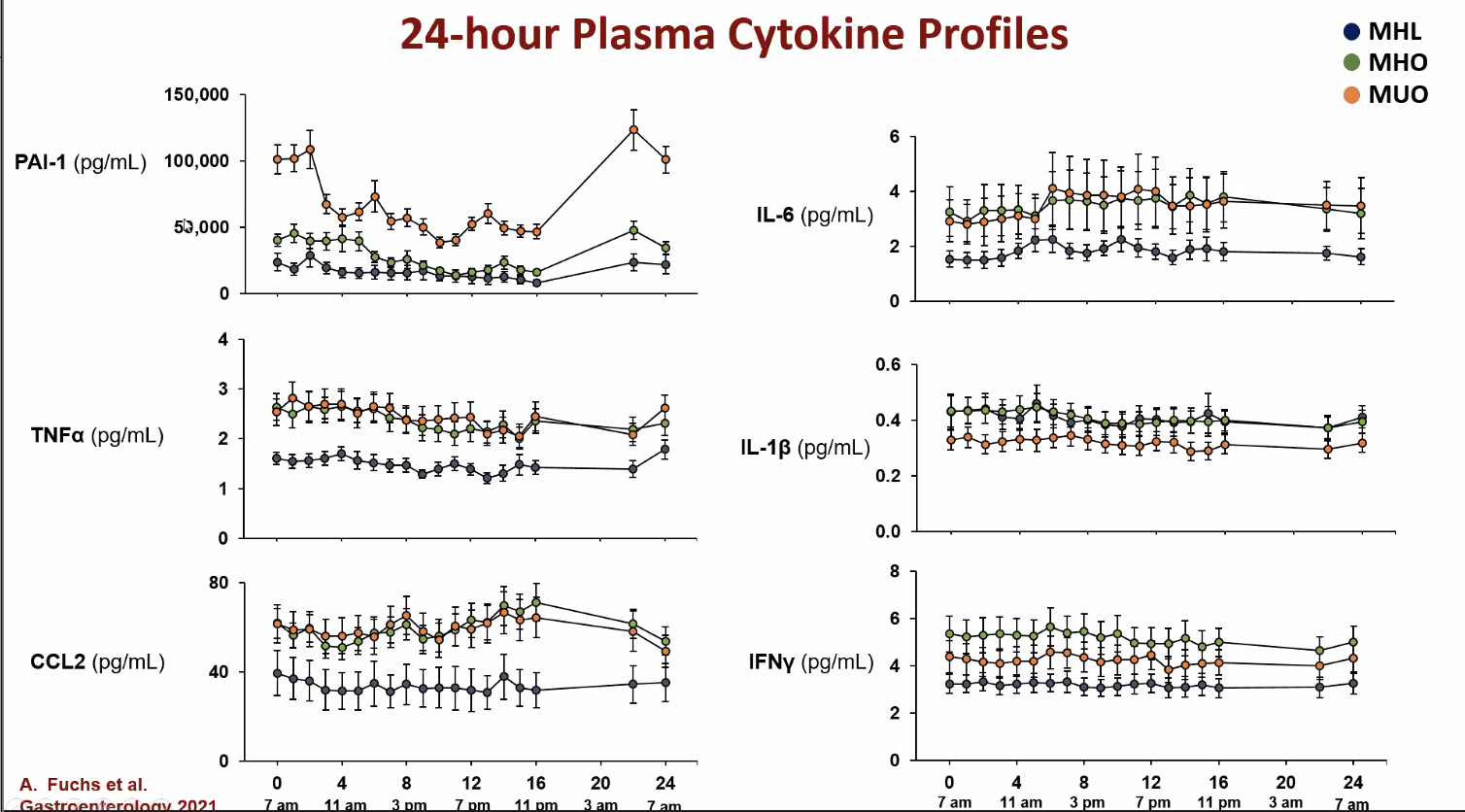
This slide shows a 24 hour measurement of various proteins in the blood. You can see how elevated PAI-1 is in the metabolically unhealthy obese.
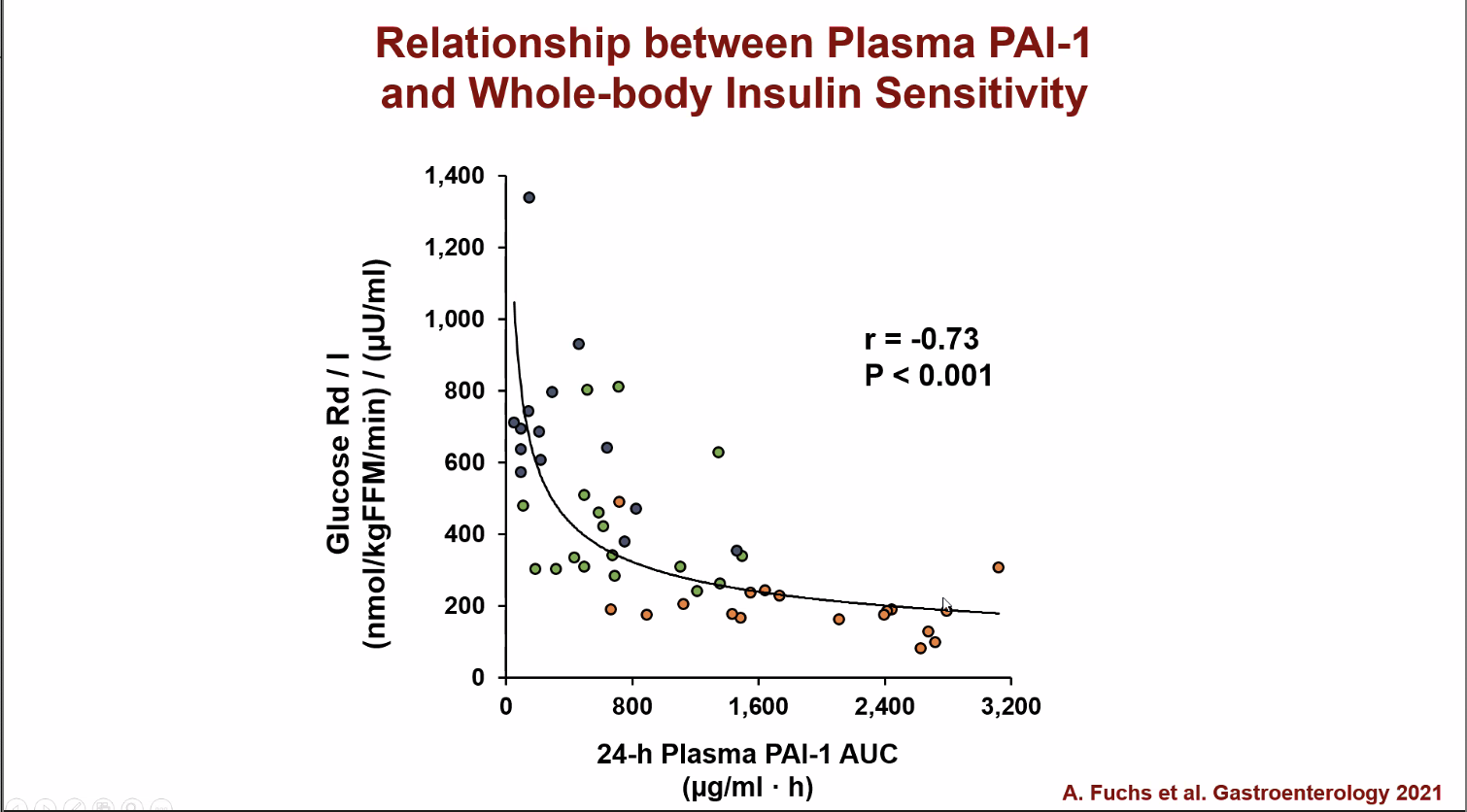
The more PAI-1 in the bloodstream, the less sensitive you are to insulin.
The metabolically unhealthy obese group had more inflammation in their belly fat than the other groups. While most inflammatory substances in the blood were similar among the groups, PAI-1, was higher in the metabolically unhealthy obese group. As we know from the previous section about low oxygen in fat tissue, higher PAI-1 is linked to poorer insulin function. Exosomes from the metabolically unhealthy obese group harmed insulin signaling in muscle and liver cells more than those from the metabolically healthy obese group.
Professor Klein emphasized that exosomes are a very hot area of research at the moment—something people feel is very promising and could result in new therapies.
Responses to weight loss
A little goes a long way
Even the slightest bit of weight loss improves health in a metabolically unhealthy obese person.
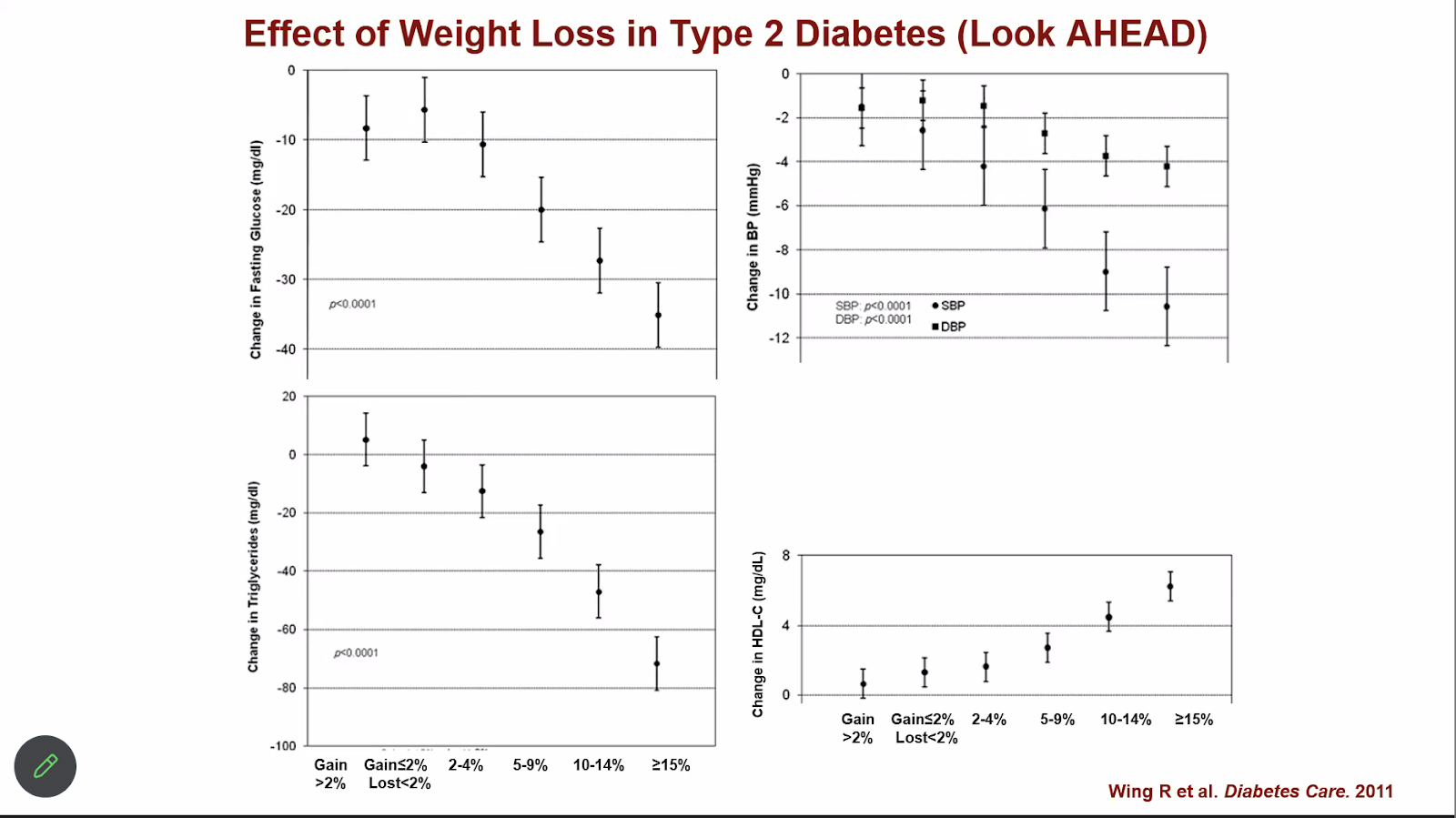
You can see positive responses to weight loss with as little as 2% to 4% weight loss, as shown by this study above done on patients with Type 2 Diabetes.
A little goes a long way, and modest improvements should not be underrated. Just as obesity negatively affects your total body health, losing weight improves everything all at once. This means you don’t need individual drugs for each obesity associated condition — you can fix a lot just by losing weight.
Liposuction isn’t a shortcut
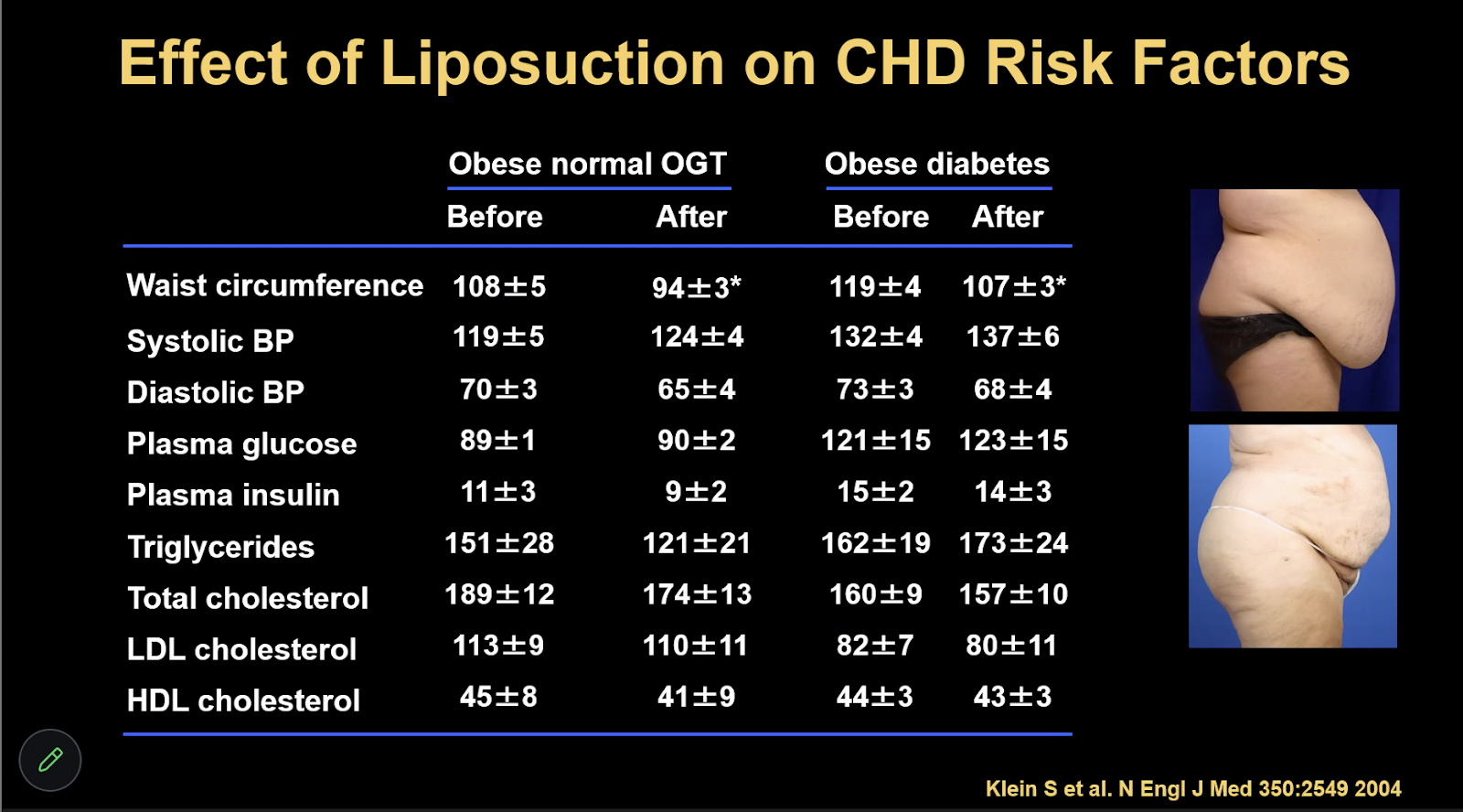
One fascinating slide showed a study in which high volume liposuction was tested—if you suck the belly fat out rather than it being lost “naturally”, can that help people? The answer was a resounding NO.
You can see in the table above that while waist circumference improved, other metrics of metabolic health like glucose and insulin levels and total cholesterol were more or less unchanged post-liposuction.
For liposuction to be effective in the long term, diet and exercise would also have to be regulated. Temporary diets do not work—lifelong, true lifestyle changes do.
What’s the best way to lose weight?
Exercise alone is not effective
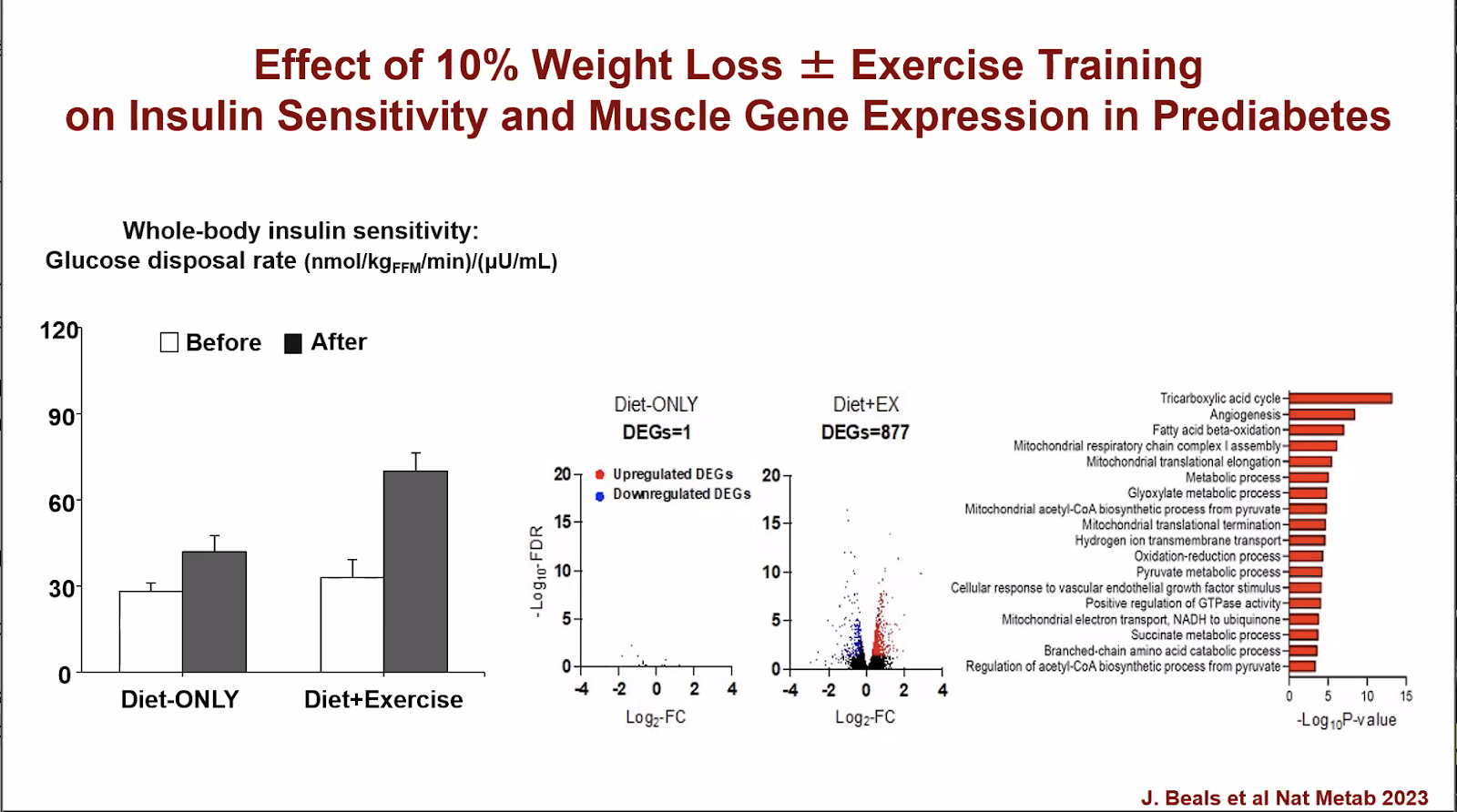
Exercise alone is not an effective weight loss strategy. While you can burn a lot of calories with exercise, you can put them all back just as easily with a very small amount of food.
That said, exercise should be included as part of a weight loss program, because it acts as a multiplier on metabolic effects—look at the difference in whole body insulin sensitivity when you combine diet and exercise!
As demonstrated by the study above, combining regular exercise with a 10% loss of body weight more than doubles sensitivity to insulin, compared with a 10% weight loss without exercise.
This study was completed by Professor Klein’s lab and in the press release for it he himself said:
“Our study involved detailed analyses of metabolic changes in muscle and body fat before and after a 10% weight loss in people who lost weight with diet therapy alone and in those who lost the same amount of weight with diet therapy plus supervised exercise training. The results demonstrate that the benefits of combining exercise with weight loss are considerable.”
GLP-1s have changed the game
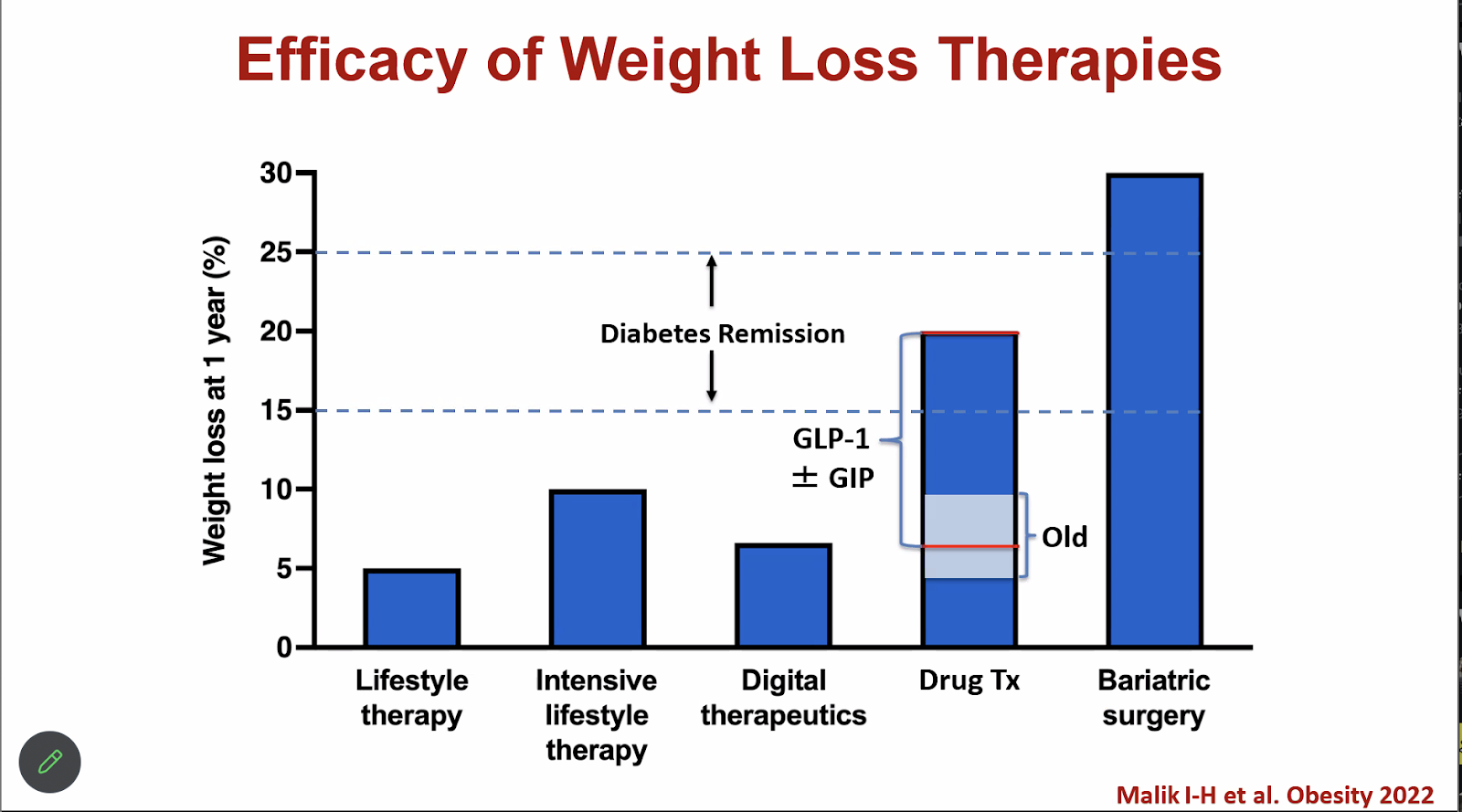
To achieve diabetes remission, you need 15%-25% total weight loss—something that’s near impossible to achieve with traditional interventions like lifestyle therapy (say, Weight Watchers or Noom).
GLP-1 drugs like Ozempic have “changed the landscape dramatically” per Professor Klein’s words in the lecture. They have made this significant amount of weight loss achievable by more people without having to resort to surgery.
Could it be nutrient load?
At the end of the lecture, one person in the audience asked a provocative question which got Professor Klein excited–”Could it be nutrient load?”
Essentially, there’s another mechanism for bad metabolic health outcomes at play when we overeat called nutrient overload. Nutrient overload suggests that we’re giving our bodies more nutrients than they can process, and long story short — that stresses them out, leading to poor health.
Our bodies do a great job smoothing over external stimuli to maintain an equilibrium—but like all systems, it has its limits. Irreversible damage can occur in the face of constant challenges—if you consistently give your body more of a nutrient than it can handle, poor health can occur as a result.
This was the first time I had been exposed to the concept of nutrient load and the idea of “overnutrition”. While it was not discussed at length during the lecture, I did want to mention it in case you, like me, had never heard of it before.
Conclusion
Obesity and its outcomes on health are not one-size-fits all! Not all individuals with obesity have the same metabolic health risks or outcomes.
While many people associate obesity with health issues like diabetes, heart disease, and high blood pressure, the reality is more complex. It is possible to be “metabolically healthy” and obese.
Clearly defining metabolically healthy obesity and studying those people in relation to the metabolically unhealthy obese and lean can hopefully continue to unlock a greater understanding of why excess weight drives so many poor health outcomes.
